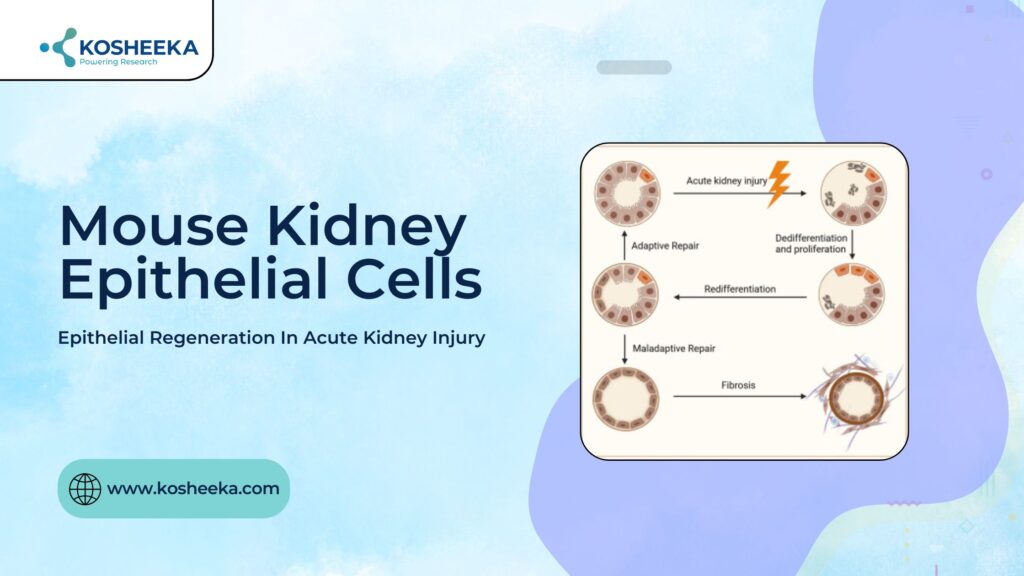
Acute kidney injury is a multifactorial disorder with high mortality risk. Lack of effective treatment has urged the scientific community to study the natural phenomenon of epidermal regeneration. Epithelial injury is linked to kidney injury, and its regeneration can prove to be a viable treatment. Therefore, much research has employed the mouse kidney primary epithelial cells to delineate the signaling pathways that lead to repair and regeneration.
Acute Kidney Injury
Acute kidney injury is a complex biochemical process causing rapid functional decline and consequent multi-organ failure. Tubular and vascular damage, along with inflammation, frequently accompany it. The blood supply is concentrated in the glomerulus, which increases the susceptibility of the kidney to injury. It usually progresses to a chronic form of the disease, leading to end-stage kidney disease that requires an organ transplant.
Epithelial regeneration
Kidney injury also incorporates epithelial cell injury. The injury can be ischemic in nature, driving ATP depletion and subsequent cell death, or occur due to toxins that lead to direct cell loss. Tubular epithelial cells are sensitive to insult owing to their high requirement for ATP for cellular transport. However, mild injury undergoes complete functional recovery due to epithelial regeneration. The debatable issue is the cell type that drives regeneration. The studies have eliminated stem cells external to renal tissue as possible candidates for the regeneration process. Two theories have cropped up supporting either the presence of renal progenitors or the transient dedifferentiation of epithelial cells, respectively.
Renal Progenitors
The cell dedifferentiation has been evident in several studies. Electron microscopy and staining techniques have revealed the loss of normal morphology of epithelium, such as brush border and infoldings, and its conversion into flattened progenitors. The intermediate filaments vimentin, keratin-7, and keratin-19 mark the progenitors.
Further research demonstrated that an epithelial cell subpopulation exists. They are smaller, wedge-shaped, less differentiated, containing a low mitochondrial content, conferring more resistance to injury, along with the high expression of Bcl-2. The expression of CD24 and CD133 further distinguishes the population. They were referred to as scattered tubular cells (STCs). They not only had the self-renewal capacity but also showed differentiation into osteocytes, neurons, and adipocytes. They reside in the Bowman’s capsule while different regions of the tubule also contain their tubular-committed progenitors.
Origins of Regeneration
Research attempts to delineate the kidney injury mechanisms exhibited the upregulation of SRY-Box transcription factor-9 (Sox9). Interestingly, instead of STCs-the progenitors, differentiated epithelial cells increased the Sox9 expression, thus contributing more to the injury repair process. Research has also suggested that fully differentiated epithelial cells remain in the GI phase of the cell cycle, ready to enter it when needed.
However, STCs have more regeneration potential. They can form tubuloid-like structures in a three-dimensional environment. STC administration in disease models showed distribution in the cortex and medulla, hinting at their involvement in regeneration. However, certain studies raise the question of whether the kidney has an existing pool of progenitors or whether these progenitors arise from the epithelium.
Strategies for Research
Several strategies focused on discovering the cell type responsible for the epithelial cell regeneration. Transgenic mouse models with an inducible reporter that tags the differentiated epithelium is one of them. It proposes that the regeneration by the progenitors will dilute the reporter signal, whereas no dilution will be noticeable if the tagged epithelial cells cause the regeneration. Additionally, cell cycle markers such as Ki-67 and BrdU were also employed. The former represents the transition from G1 to the mitotic phase, while the latter marks the proliferation. However, all the strategies have their inherent drawbacks, turning the conclusive results into doubts.
In Vitro Research
In vitro research has been crucial for deciphering the pathways behind disease and regeneration. Two-dimensional cell culture has employed primary mouse renal epithelial cell and cell lines. They are beneficial to study the transport of multidrug-resistant substrates and to screen drugs for efficacy and toxicity. Among the primary cells, proximal tubular epithelium and embryonic kidney cells are the most common in vitro models. M-1, TKPTS, and iBMK are a few immortalized mouse kidney epithelial cells
available for research.
Conclusion
The molecular processes that propel the regeneration process have been under investigation. Research has indicated that the dedifferentiation of fully differentiated epithelial cells and the presence of renal progenitors both aid in the healing process. Some reports affirm the progenitor-based repair, while others hold the dedifferentiated cells responsible. However, each study has its limitations. The real drivers of the process remain hidden, and so do the pathways. Mouse models have substantially advanced the knowledge of the field, with the ability to create transgenic models and the availability of mouse kidney primary cells and immortalized mouse kidney epithelial cell lines. A robust experimental design can answer the question: Which cells mediate regeneration? It can be the key to unlock the treatment for acute kidney injury. Kosheeka offers mouse kidney primary epithelial cells to boost your research and accelerate drug discovery.




